May 5, 2022
What are the 340B Discount Drug Program Requirements?
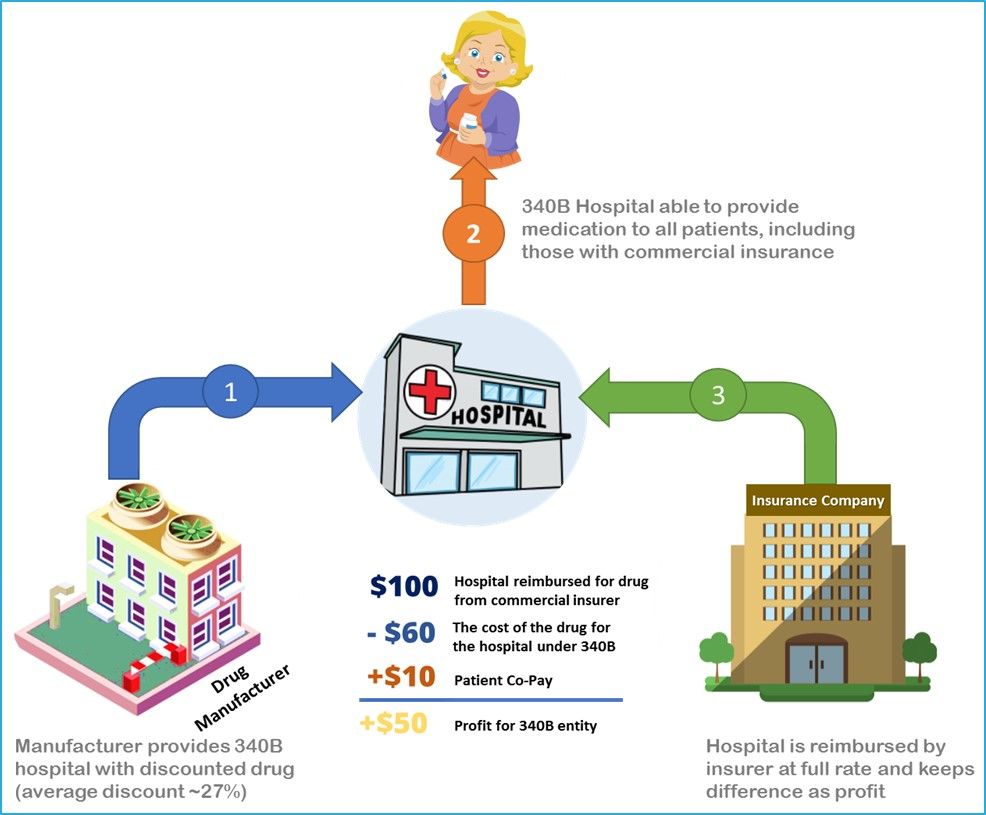
In 1992, Congress created a federal drug discount program under Section 340B of the Public Health Service Act. Section 340B, or the 340B discount drug program, requires pharmaceutical manufacturers that participate in Medicaid and Medicare Part B to give discounts on covered outpatient drugs that are purchased by participating hospitals and organizations.
For hospitals to qualify for the 340B discount drug program, they must meet the following three requirements.
1.Government ownership
The hospital must be owned or operated by a unit of the state or local government, be a public or private non-profit organization which has been formally granted governmental powers by state or local government, or be a private non-profit hospital with government contracts to provide healthcare services to low-income patients that are not covered under Medicare or Medicaid.
2.Medicare Disproportionate Share Hospital (DSH)
The hospital must have a sufficient Medicare disproportionate share hospital adjustment percentage that is greater than 11.75 percent for the most recent fiscal year. Your hospital’s DSH adjustment depends on the number of inpatient days of its Medicaid and Supplemental Security Income patients.
3.Written Agreement
If your organization meets the first two requirements to participate in the 340B program, then it’s time to sign a written agreement that you will not obtain covered outpatient drugs through a group purchasing organization (GPO). Any pharmacy located at your hospital’s main address and reimbursable on your Medicare cost report may purchase and dispense the discounted drugs for outpatient use. The 340B discounted drugs may be dispensed through an outpatient pharmacy or in a clinical setting.











